Ankylosing Spondylitis And Axial Spondyloarthritis
Spondyloarthritis is a term used to include a group of arthritis-associated conditions. One of the associated conditions include axial spndyloarthritis , which is a potentially disabling inflammatory arthritis that typically affects the spine however, may also affect joints in the arms and legs. Symptoms typically begin before the age of 45. Those with axSpA can be further classified into 2 subtypes such as ankylosing spondylitis and nonradiographic axial spondyloarthritis . Individuals affected by AS exhibit radiographic abnormalities consistent with sacrolitis, whereas, findings are absent or minimal on plain radiography for those with nr-axSpA. Individuals with nr-axSpA may have evidence of active inflammation of the sacroiliac joints on MRI findings . The diagnosis of axSpA, including AS and nr-axSpA, should be considered in persons with continuous chronic back pain prior to age 45, Per UpToDate, “there is no single historical feature, physical finding, laboratory test, or imaging study with sufficient specificity by itself to establish the diagnosis without the presence of additional abnormalities. Thus, the presence of a combination of features together with the exclusion of other diagnoses that may explain such symptoms or findings is necessary to arrive at an accurate diagnosis” .
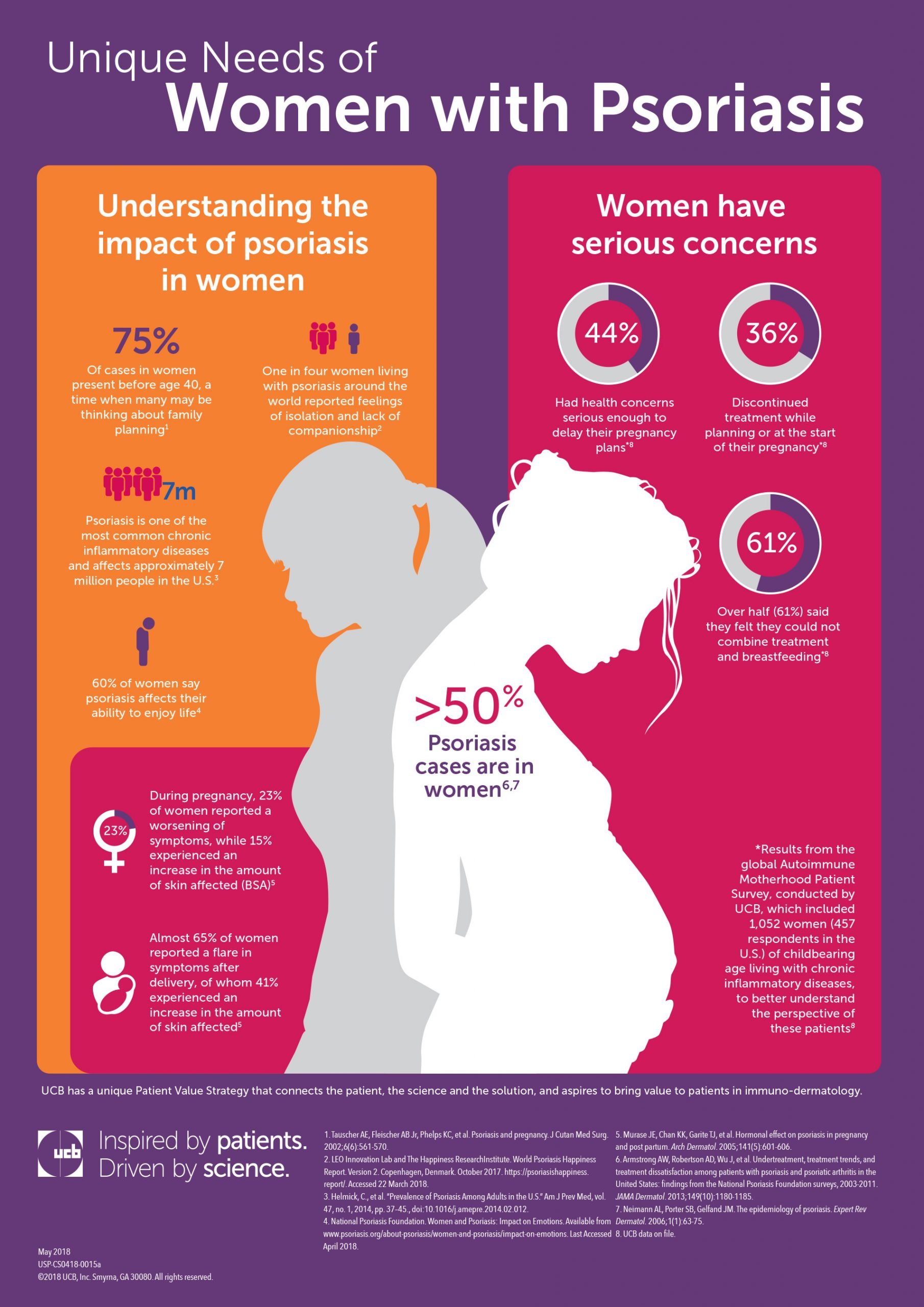
Cimzia Shows Promise In Treatment Of Psoriasis
Significant positive results in 12-week phase II trial
BRUSSELS , July 18, 2006 7:00 am CET UCB today announced significant positive results from the first study to evaluate the efficacy and safety of CIMZIA , a new type of anti-tumour necrosis factor therapy, in the treatment of patients with moderate to severe psoriasis.
The phase II, randomized, double-blind, placebo-controlled dose-ranging study involved 176 patients with moderate to severe chronic plaque psoriasis who were candidates for systemic therapy and/or photo- or photochemo-therapy. The patients were randomized to one of two different subcutaneous dosing regimens using the liquid formulation: CIMZIA 400mg given every other week, or an initial dose of CIMZIA 400mg followed by a dose of CIMZIA 200mg given every other week or placebo.
The co-primary endpoints for the study were achieved with a high degree of statistical significance, including the proportion of patients achieving a 75% decrease from baseline in Psoriasis Area and Severity Index score following treatment at week 12:
Percentage of patients achieving PASI 75 at week 12:
Placebo – 6.8
CIMZIA 200mg – 74.6
A 24-week follow-up period to this study is currently ongoing, with a retreatment study also in progress. Data from the CIMZIA psoriasis clinical trial programme will be presented at forthcoming dermatology congresses.
References
1 National Psoriasis Foundation. www.omni.ac.uk/browse/mesh/DO11565.html. Accessed 4 July 2006.
Takeaway And Helpful Resources
The dosages in this article are typical dosages provided by the drug manufacturer. If your doctor recommends Cimzia, they will prescribe the dosage thats right for you. Always follow the dosage that your doctor prescribes for you.
As with any drug, never change your dosage of Cimzia without your doctors recommendation. If you have questions about your dosage, talk with your doctor.
Besides learning about dosage, you may want other information about Cimzia. These additional articles might be helpful:
- More about Cimzia. For information about other aspects of Cimzia, refer to this article.
- Side effects. To learn about side effects of Cimzia, see this article. You can also look at the Cimzia medication guide.
- Drug comparison. To find out how Cimzia compares with Humira, read this article.
- Details on your condition. For details on arthritis, visit our arthritis hub. You can also see our lists of related articles for information on your condition:
Read Also: How To Cure Psoriasis From The Inside Out
Cimzia 200 Mg Solution For Injection In Pre
This information is intended for use by health professionals
Cimzia 200 mg solution for injection in pre-filled syringe
Each pre-filled syringe contains 200 mg certolizumab pegol in one ml.
Certolizumab pegol is a recombinant, humanised antibody Fab’ fragment against tumour necrosis factor alpha expressed in Escherichia coli and conjugated to polyethylene glycol .
For the full list of excipients, see section 6.1.
Clear to opalescent, colourless to yellow solution. The pH of the solution is approximately 4.7.
Rheumatoid arthritis
Cimzia, in combination with methotrexate , is indicated for:
the treatment of moderate to severe, active rheumatoid arthritis in adult patients when the response to disease-modifying antirheumatic drugs including MTX, has been inadequate. Cimzia can be given as monotherapy in case of intolerance to MTX or when continued treatment with MTX is inappropriate
the treatment of severe, active and progressive RA in adults not previously treated with MTX or other DMARDs.
Cimzia has been shown to reduce the rate of progression of joint damage as measured by X-ray and to improve physical function, when given in combination with MTX.
Axial spondyloarthritis
Cimzia is indicated for the treatment of adult patients with severe active axial spondyloarthritis, comprising:
Ankylosing spondylitis
Adults with severe active ankylosing spondylitis who have had an inadequate response to, or are intolerant to nonsteroidal anti-inflammatory drugs .
Psoriatic arthritis
Is Cimzia Used For Ulcerative Colitis If So Whats The Dosage
The Food and Drug Administration hasnt approved Cimzia to treat ulcerative colitis . But Cimzia is being studied to see if it could be effective for this use.
Because Cimzia isnt approved to treat ulcerative colitis, there isnt a recommended dosage for this condition. If youre interested in taking Cimzia to treat ulcerative colitis, talk with your doctor. This would be an off-label use of the drug.
Read Also: How To Stop A Psoriasis Flare Up
Cimzia Is A Pegylated Fc Free Anti
Cimzia is the first PEGylated, FC free anti-TNF indicated for the treatment of severe active Axial Spondyloarthritis, active Psoriatic Arthritis, moderate to severe Plaque Psoriasis, and moderate to severe Rheumatoid Arthritis inadequately controlled with prior therapy.1,6,10-12Cimzia’s licensed indications include severe active Axial Spondyloarthritis , active Psoriatic Arthritis , moderate to severe Plaque Psoriasis , and moderate to severe Rheumatoid Arthritis inadequately controlled with prior therapy.1
Frequently Asked Questions About Cimzia
How long do I have to take Cimzia ?
There isn’t a specific amount of time that you’ll need to take Cimzia . Your provider will likely have you continue taking the medication as long as it’s helping your condition and you’re not having side effects.
Where should I inject Cimzia ?
Cimzia is administered through an injection under the skin of your upper thighs or stomach into the fat tissue between your skin and muscle. If you’re giving the injection into your stomach, make sure it’s at least 2 inches away from your belly button. Never inject the medication into your muscle or vein. To lower the risk of bruising and irritation, alternate injection sites every day. Each injection should be given at least 1 inch away from the site you used the previous day. Don’t inject into skin that is tender, bruised, red or hard, or where there are scars or stretch marks.
Can I receive vaccines if I’m taking Cimzia ?
Yes, you can still receive vaccines, including the flu shot, while taking Cimzia . However, you should not receive live vaccines because it’s not known if they are safe while you’re taking Cimzia . Examples of live vaccines include the live flu vaccine , which is given through a nasal spray, and the measles, mumps, rubella vaccine. Let your provider or pharmacist know that you’re taking Cimzia before you receive any vaccines.
Can Cimzia be used in children?
Can I use Cimzia if I’m pregnant?
Which medications should I not take with Cimzia ?
Read Also: Coconut Oil To Treat Scalp Psoriasis
What If I Miss A Dose
If you miss an appointment to have an injection of Cimzia, call your doctors office right away to reschedule.
If you usually inject Cimzia yourself and you miss a dose, call your doctors office to find out what to do. They may tell you to take the missed dose as soon as possible. But if its nearly time for your next dose, they may tell you to skip the missed dose.
Your doctor can also tell you if you need to adjust your dosing schedule after a missed dose.
If you need help remembering to take your dose of Cimzia on time, try using a medication reminder. This can include setting an alarm, downloading a reminder app, or setting a timer on your phone. It can also be helpful to write your Cimzia dosing schedule on a calendar.
The dosage of Cimzia youre prescribed may depend on:
- the type and severity of the condition youre using Cimzia to treat
- your body weight
- how you respond to the treatment
If you have any questions about the dosage of Cimzia you should take, talk with your doctor.
How Does Cimzia Work
In autoimmune diseases like psoriasis, the immune system is abnormally triggered and causes an increase in inflammation, which causes the inflamed patches on the skin. Cimzia targets and blocks tumor necrosis factor , a protein involved in the inflammatory response. By blocking TNF, Cimzia can reduce the symptoms of psoriasis. Cimzia is a PEGylated TNF inhibitor. PEG is polyethylene glycol, a substance that is attached to a protein to make it stay in the body longer.
Also Check: Light Therapy For Psoriasis At Home
If My Cimzia Dose Requires Two Injections Can I Use The Same Spot For Both
No, you should not use the same spot for a Cimzia dose of two 200-milligram injections.
Instead, you should use a different area for each injection. For example, if your first injection is in your upper thigh, your second injection should be at least 1 inch away.
For more information on where you can inject Cimzia, see the How to use section below.
The Cimzia dosage your doctor prescribes will depend on several factors. These include:
- your body weight
- how well Cimzia works to treat your condition
- side effects that you may have from using Cimzia
If you have questions about other factors that can affect your dosage of Cimzia, talk with your doctor.
Fda Expands Approval Of Cimzia For Moderate
We were unable to process your request. Please try again later. If you continue to have this issue please contact .Emmanuel Caeymaex
The FDA has approved a label extension for UCBs TNF inhibitor Cimzia to include an indication for adults with moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy, according to a company press release.
Cimzia is the first Fc-free biologic of its kind approved by the FDA to treat this challenging skin condition, building on 10 years of market experience with demonstrated efficacy and established safety across multiple inflammatory diseases,Emmanuel Caeymaex, head of immunology and executive vice president of the immunology patient value unit at UCB, said in the release. This approval reflects our heritage of making a difference for specific patient populations with unmet needs, and we are especially gratified to welcome immuno-dermatology patients for the first time to our community of support.
Caeymaex added that the approval of Cimzia for psoriasis, as well as its recent label update regarding pregnancy and breastfeeding in women with chronic inflammatory diseases, are important treatment advances.
UCB is committed to improving care for psoriasis patients and is also investigating bimekizumab, a therapy with significant potential for psoriasis patients, he said in the release.
Also Check: What Does Psoriasis Scalp Look Like
Us Food And Drug Administration
- Reducing signs and symptoms of Crohns disease and maintaining clinical response in adult patients with moderately to severely active disease who have had an inadequate response to conventional therapy
- Treatment of adults with moderately to severely active rheumatoid arthritis
- Treatment of adult patients with active psoriatic arthritis
- Treatment of adults with active ankylosing spondylitis
- Treatment of adults with active non-radiographic axial spondyloarthritis with objective signs of inflammation
- Treatment of adults with moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy
Cimzia carries a Black Box Warning for serious infections and malignancy. These include:
- Increased risk of serious infections leading to hospitalization or death including tuberculosis , bacterial sepsis, invasive fungal infections , and infections due to other opportunistic pathogens
- Cimzia should be discontinued if a patient develops a serious infection or sepsis
- Perform test for latent TB if positive, start treatment for TB prior to starting Cimzia
- Monitor all patients for active TB during treatment, even if initial latent TB test is negative
- Lymphoma and other malignancies, some fatal, have been reported in children and adolescent patients treated with TNF blockers, of which Cimzia is a member
- Cimzia is not indicated for use in pediatric patients.
Most common adverse reactions include upper respiratory tract infection, rash, and urinary tract infection.
What Other Drugs Could Interact With This Medication

There may be an interaction between certolizumab pegol and any of the following:
- BCG
- immunosuppressants
- leflunomide
- medications that weaken the immune system
- other biologics
- roflumilast
- siponimod
- vaccines
If you are taking any of these medications, speak with your doctor or pharmacist. Depending on your specific circumstances, your doctor may want you to:
- stop taking one of the medications,
- change one of the medications to another,
- change how you are taking one or both of the medications, or
- leave everything as is.
An interaction between two medications does not always mean that you must stop taking one of them. Speak to your doctor about how any drug interactions are being managed or should be managed.
Medications other than those listed above may interact with this medication. Tell your doctor or prescriber about all prescription, over-the-counter , and herbal medications you are taking. Also tell them about any supplements you take. Since caffeine, alcohol, the nicotine from cigarettes, or street drugs can affect the action of many medications, you should let your prescriber know if you use them.
All material copyright MediResource Inc. 1996 â 2021. Terms and conditions of use. The contents herein are for informational purposes only. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Source: www.medbroadcast.com/drug/getdrug/Cimzia
Don’t Miss: Are Tanning Beds Good For Psoriasis
What Are The Risks Of Biosimilars
The risks and side effects of biosimilars are the same as those associated with their biologic reference product. Anyone considering taking a biosimilar should talk with their health care provider about the short- and long-term side effects and risks. It is important to weigh the risks against the benefits.
Biologics and biosimilars act on cytokines, which are specific proteins released by the immune system that can cause inflammation. Biologics suppress the function of the overactive immune system. When on a biologic or biosimilar, you may have a higher risk of infection. If you develop any signs of an infection, contact your health care provider right away.
Signs of infection include:
- Damp, sticky feeling or sweating
- Fever
Preparation And Administration Of Cimzia Using The Prefilled Syringe
After proper training in subcutaneous injection technique, a patient may self-inject with the CIMZIA Prefilled Syringe if a physician determines that it is appropriate.
Patients using the CIMZIA Prefilled Syringe should be instructed to inject the full amount in the syringe , according to the directions provided in the Instructions for Use booklet.
Also Check: Guttate Psoriasis Vs Pityriasis Rosea
Brand Selection For Medically Necessary Indications
As defined in Aetna commercial policies, health care services are not medically necessary when they are more costly than alternative services that are at least as likely to produce equivalent therapeutic or diagnostic results. Cimzia is more costly to Aetna than other targeted immune modulators for certain indications. There is a lack of reliable evidence that Cimzia is superior to other lower cost targeted immune modulators. Therefore, Aetna considers Cimzia to be medically necessary only for members who have a contraindication, intolerance, or ineffective response to the available equivalent alternative targeted immune modulators for the following medically necessary indications per criteria below:
Monitoring To Assess Safety
Before initiation of therapy with CIMZIA, all patients must be evaluated for both active and inactive tuberculosis infection. The possibility of undetected latent tuberculosis should be considered in patients who have immigrated from or traveled to countries with a high prevalence of tuberculosis or had close contact with a person with active tuberculosis. Appropriate screening tests should be performed in all patients.
Don’t Miss: Il 17 Inhibitors For Psoriasis
Serious Or Deadly Infections
- Risk factors: Active, chronic, or recurrent infection | Age 65 years and older | Having other conditions that raise your risk of infection | Living in or traveling to places with high rates of TB or fungal infections | Taking medications that weaken your immune system
Cimzia can affect your body’s ability to fight off infections, which can raise your risk of serious or life-threatening bacterial, viral, or fungal infections. The risk is higher for people who have an active infection, people who have infections that keep coming back, adults aged 65 years and older, people with certain chronic conditions that make it easier to develop infections, and people taking medications that weaken your immune system .
Cimzia can also cause new or old tuberculosis infections to come back. Similarly, the medication can reactivate the hepatitis B virus if you’re a carrier of the virus. For these reasons, you’ll need to get tested for both TB and HBV before starting Cimzia and get treatment if you test positive.
If you develop symptoms of an infection while taking Cimzia , go to the hospital right away so you can be tested for an infection. You might have to stop taking Cimzia so your body can fight the infection. Tell your provider about your medical conditions and medications so they can make sure Cimzia is safe for you.
What To Do In Case You Use Too Much Cimzia
Call your doctor right away if you think youve used too much Cimzia. You can also call 800-222-1222 to reach the American Association of Poison Control Centers, or use its online resource. But if you have severe symptoms, call 911 or your local emergency number immediately, or go to the nearest emergency room.
Also Check: Scalp Psoriasis Flare Up Treatment