Power And Sample Size Analyses
With this margin, one-sided testing and a randomization ratio of 2:1 DR versus UC, we calculated that 222 patients need to be included to reject the null-hypothesis of inferiority. Taking a drop-out rate of 10% into account, 244 patients need to be included, with 162 in the DR arm and 82 in the UC arm.
Eular Guidelines Point To Bdmards
Overall, IL-17 inhibitors have exhibited substantial benefit in managing both articular and extraarticular disease in patients with PsA. The 2018 ACR guidelines were not able to include the newer IL-17 trials into their consensus recommendations and current evidence recommends TNFa-I as the preferred bDMARD, however the European agency EULAR released a 2019 update to address these new studies.5,6 In the updated EULAR guidelines, the current recommendation is that all bDMARDs are viable options with no preference for one over the other. IL-17 Inhibitors were elevated to have the same recommendation as TNFa-I and IL-12/23 inhibitors. The EULAR guidelines mention evaluating individual components of each agent on a case-by-case basis, and specifically note that IL-17 inhibitors should especially be considered in those with relevant skin involvement.5
Clinical trials support bDMARDs respective place in therapy as first-line treatment options to reduce pain and improve activities of daily living due to better toleration in individuals with psoriatic arthritis.
Acknowledgements: The authors thank Ashley Jackson, Pharm D, for her technical editing and clinical expertise in the drafting of this manuscript.
Experimental And Clinical Evidence For A Role Of Il
First antibodies that target IL-17A or its receptor IL-17RA are approved for the treatment of psoriasis. Secukinumab and ixekizumab both neutralize IL-17A, while brodalumab binds to the IL-17RA. The clinical efficacy of these IL17-targeting antibodies in psoriasis is demonstrated in several randomized studies and registries, as a clinical improvement of at least 75% as measured by the PASI is observed in > 80% of patients treated . Similar PASI responses are achieved in clinical trials testing antibodies neutralizing IL-23 , which is a potent inducer of IL-17A production by immune cells . In the recent five years there has been growing clinical evidence that IL-17A inhibitors are effective and safe in treating moderate to severe plaque type psoriasis.
Ixekizumab is a humanized monoclonal antibody with a selective inhibition of IL-17A and approved for the treatment of moderate to severe psoriasis. After initial s.c. treatment with 160 mg, followed by 80 mg every other week in the first 12 weeks, ixekizumab showed similar high PASI 90 responses as other IL-17 inhibitors in direct comparison to etanercept and ustekinumab . Also, long-term 4-year continuous treatment with ixekizumab has recently been presented . Ixekizumab has also received first line approval from the EMA, but due to its late approval date has not yet been included in the most recent guidelines.
Clinical studies with anti-IL-17 are ongoing in adolescents and children, and results are not yet presented.
Read Also: Light Therapy For Psoriasis Cost
Data Collection And Management
All data will be collected and entered in Castor, an electronic data management system, which is setup for clinical trials . Data will be coded and kept based on the rules for good clinical practice by GCP-certified personnel . Handling of personal data will comply with the General Data Protection Regulation . All blood samples will be coded before sending to Ghent University Hospital for storage and to University Hospitals Leuven where the samples will be analyzed.
Demonstrated Efficacy In Phase 3 Trials
Secukinumab
Secukinumab was further evaluated in four Phase 3 randomized, double-blind, placebo-controlled trials two 52-week trials, ERASURE and FIXTURE 12, one 12-week trial, FEATURE, a bridging study assessing response of self-administration by pre-filled syringe13, and SCULPTURE, a 52-week trial comparing fixed-dose vs. retreatment-as-needed maintenance regimen with 150 mg or 300 mg secukinumab. The SCULPTURE trial is ongoing and results have not yet been published.14
In the ERASURE study, 738 patients were randomly assigned to SC secukinumab at a dose of 300 mg or 150 mg or placebo.12
In the FIXTURE study, 1,306 patients were randomly assigned to SC secukinumab at a dose of 300 mg or 150 mg , etanercept at a dose of 50 mg or placebo.12
Co-primary endpoints at week 12 of PASI 75 and a score of 0 or 1 on a 5-point modified investigators global assessment were met by a higher proportion of patients treated with secukinumab vs. those treated with etanercept or placebo.12
The proportion of patients meeting PASI 75 at week 12 was higher with both secukinumab doses than with placebo or etanercept. In the ERASURE study, the rates were 81.6% with secukinumab 300 mg, 71.6% with secukinumab 150 mg, and 4.5% with placebo in the FIXTURE study, the rates were 77.1% with secukinumab 300 mg, 67.0% with secukinumab 150 mg, 44.0% with etanercept, and 4.9% with placebo .12
Ixekizumab
Brodalumab
Recommended Reading: Scalp Psoriasis Flare Up Treatment
Evidence Mounts For Il
A paradigm shift is underway in the treatment of psoriasis. Two newer classes of biologics, the interleukin 23 and interleukin 17 inhibitors are challenging the longstanding supremacy of tumor necrosis factor inhibitors like Humira , Enbrel , and Remicade . The innovative agents bring new mechanisms of action and superior results in clearing the ugly patches of scaly skin that characterize psoriasis, keeping skin clear longer, and working for patients who were considered nonresponders.
The IL-23 inhibitors include Stelara , Tremfya , Ilumya and Skyrizi while the IL-17 inhibitors include Cosentyx , Taltz and Siliq . A growing number of head-to-head trials are showing that the IL inhibitors are superior to the TNF inhibitors, although the results must be viewed with some caution because much of the research has been funded by manufacturers.
IL-23s vs. TNF inhibitors
Manufacturers of the IL-23 inhibitors have been particularly aggressive in trying to differentiate their wares from the TNF inhibitors. In Janssens VOYAGE 1 study, for example, Tremfya was matched up against Humira. At the end of 48 weeks, 76.3% of Tremfya patients achieved PASI 90 compared to 47.9% of Humira patients. The PASI 90 stands for psoriasis area and severity index 90, a standard outcome measure in psoriasis clinical trials that means there has been a 90% improvement in skin plaque.
IL-23 vs. IL-17
IL-23 may be the best target
Related Stories
Other Types Of Biologics
Biologics refer to any type of medical treatment that is derived from living organisms. They can include a wide variety of therapeutic options such as blood platelets, hormones, vaccines, and stem cells. Generally, biologic medications for treating autoimmune conditions involve using antibodies to directly target autoimmune processes to decrease inflammation.
Read Also: Psoriasis Home Treatment In Telugu
Onset Of Action For Interleukin
- Journal of the European Academy of Dermatology and Venereology: JEADV
You’ve saved your first item
You can find your saved items on your dashboard, in the “saved” tab.
You’ve recommended your first item
Your recommendations help us improve our content suggestions for you and other PracticeUpdate members.
You’ve subscribed to your first topic alert
What does that mean?
Systemic Effects Of Il
These first studies investigated the effects of IL-17 on different targets involved in the local manifestations of diseases . The studies on these local effects have been based on targeting IL-1, TNF, IL-6, and now IL-17 through biological therapies., More recently, the same chronic diseases were discovered to share an increased risk of cardiovascular events.
As for other inflammatory cytokines, studies on IL-17 have focused on these new but rather forgotten targets. Regarding the local features described above, it is important to consider that cytokines interact as a team in blood and tissues .
Fig. 2
Systemic effects of IL-17 in combination with other proinflammatory cytokines. Regarding local effects, the key monocyte-derived cytokines TNF and IL-1 act on stromal cells to induce the production of IL-6 and chemokines. This effect is amplified by IL-17, often through synergistic interactions. These effects act on the brain, bone marrow, vessels, liver, and muscles. This figure depicts the overall clinical picture for many chronic and acute conditions, including the cytokine storm in COVID-19
Recommended Reading: What Causes Psoriasis In Children
Eczema In Psoriasis Patients Treated With Il
A low frequency of eczema as an adverse reaction in patients with psoriasis treated with ixekizumab, a selective interleukin -17 inhibitor, was seen according to findings in a research letter published in British Journal of Dermatology.
Investigators conducted an analysis of 13 clinical studies to evaluate the frequency, clinical subtypes, predisposing factors, time of onset, and treatment discontinuations in moderate to severe psoriasis patients who developed eczematous reactions after treatment with ixekizumab compared to treatment with etanercept, ustekinumab and placebo.
Of 5930930 patients treated with ixekizumab for up to 5 years in a clinical setting included in the analysis, 6.1% patients had treatment-emergent adverse events categorized as eczema or its clinical variants. Of these, 92.8% were younger than 65 years of age, 67.9% were men, and 80.1% were White. The study authors reported that baseline characteristics were representative of the entire study population. Also, 21.6% patients had previous biologic treatment and 74 20.5% with a history of atopy and/or allergic disease at the time of ixekizumab treatment initiation.
Investigators did not find a significant difference when comparing overall incidence rates per 100 patient-years for TE-AEs between ixekizumab, etanercept, ustekinumab or placebo.
The analysis limitations included possible misdiagnosis or reporting error for eczematous reactions.
Pathogenesis Of Plaque Psoriasis
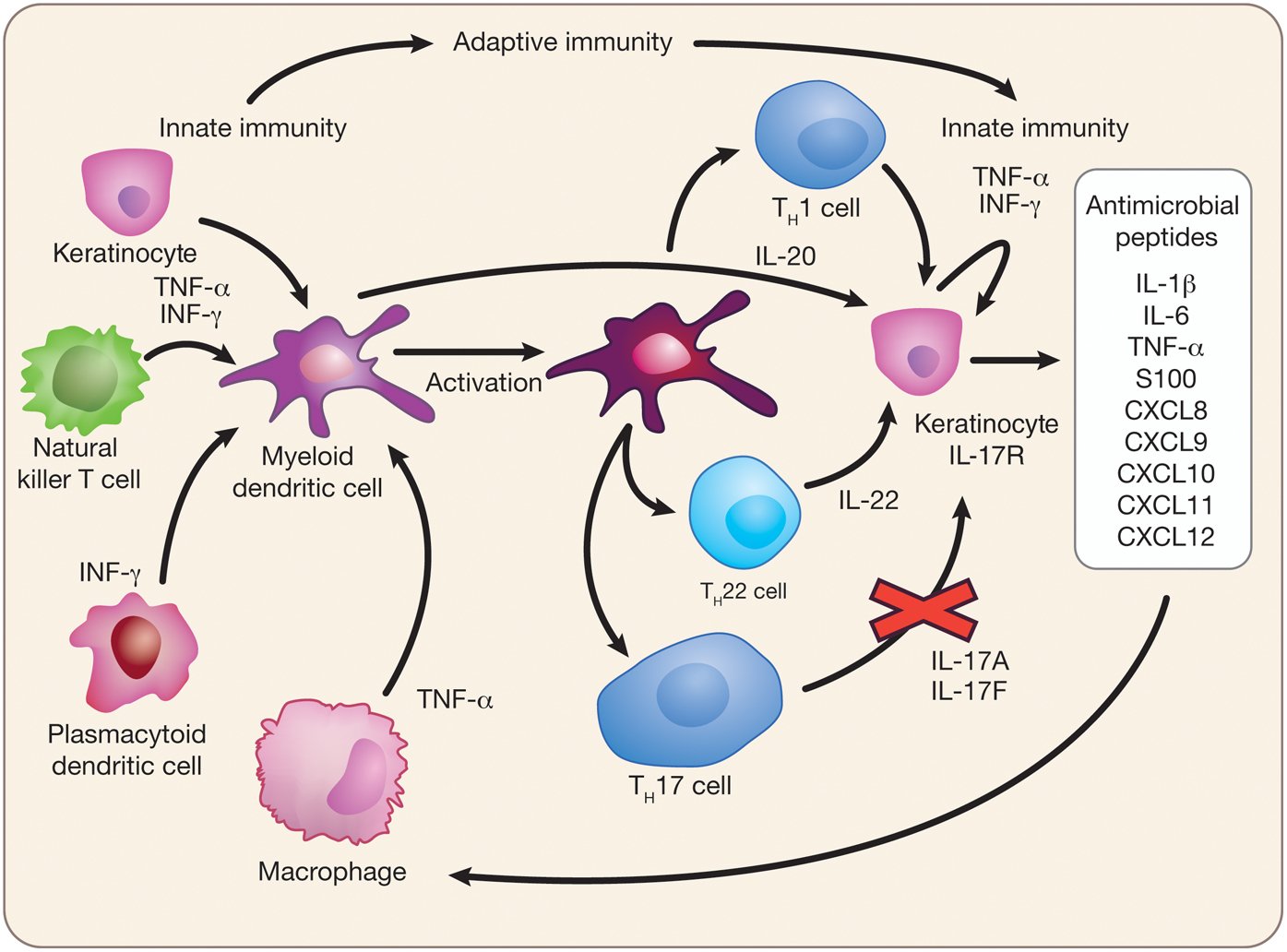
Psoriasis vulgaris is a chronic, inflammatory, immune-mediated disease.1 Genetically-susceptible individuals subjected to various environmental factors develop inflammation and subsequent keratinocyte proliferation.2
In psoriasis both the innate and adaptive immune system are dysregulated.3 The current pathogenic model in psoriasis highlights the role of T helper 17 /interleukin 17 axis dysfunction as an important source of inflammation.3,4 Initially, activated dendritic cells may drive the activation of a subgroup of T cells through IL-23, producing IL-17, interferon- and other proinflammatory cytokines. This array of cytokines results in keratinocyte and vascular response changes.3-5 A consequence of keratinocyte response to the proinflammatory cytokines is production of chemokines and cytokines, which enhance cell recruitment. Specifically, neutrophil recruitment creates a positive feedback loop.4,6 Because of its potential action on keratinocytes, IL-17 has been considered a driver cytokine in psoriasis.3,7 Treatments targeting the IL-17 pathway using monoclonal antibodies have shown significant clinical efficacy in reducing the inflammation in this condition.3,4,8-14
Psoriasis affects 2-3% of the population worldwide15, and it is associated with significant impairment of quality of life and work productivity16. Multiple efforts are being made to better understand its pathogenesis and to develop treatments with specific targets.
Read Also: What Is Guttate Psoriasis Pictures
Ethics Approval And Consent To Participate
The study will be performed in accordance with the applicable national laws and regulations , the guidelines and guidance documents specifying Good Clinical Practice and guidelines of competent national authorities all applicable laws, rules, and legislation in relation to clinical trials, data protection and the processing of personal data, and patients rights, including but not limited to the General Data Protection Regulation 2016/679 , the Medical Research Involving Human Subjects Act for the Netherlands, and the Belgian law relating to experiments in humans dated May 7, 2004). For the Netherlands, the Medical Ethical Committee , the competent authority , as well as all ethical bodies of each participating site, approved the study. In Belgium, the study was approved by the competent authorities and the Ethics Committee of Ghent University Hospital and University Ghent after consulting the Ethics Committees of each participating Belgian site. The trial protocol was developed according to the Standard Protocol Items: Recommendations for Interventional Trials statement. Written informed consent will be obtained from each participant. Participants should give their permission for reuse of their data and biological specimens within the written informed consent form.
Potential Therapeutic Implications Of Anti
From a therapeutic perspective, preliminary data show that pharmacological inhibition of cytokines is associated with a significant improvement in endothelial function: for instance, a single subcutaneous injection of the TNF- inhibitor etanercept is associated with an improvement in flow-mediated dilation in postmenopausal women , and a preliminary study reported a significant improvement in resting endothelial function after therapy of psoriasis with anti-TNF- and anti-IL-12p40, suggesting an activation of resting endothelial function in response to anti-inflammatory therapy . Whether this also occurs with a specific inhibition of IL-17A was tested in the CARIMA study described above, which showed a significant improvement after 54, but not 12, weeks of therapy with secukinumab . In sum, psoriasis and vascular disease appear to share common pathogenetic mechanisms, and an anti-inflammatory therapy appears to not only reduce the skin manifestations, but also to improve vascular function.
Recommended Reading: How To Stop Plaque Psoriasis
New Therapeutic Options For The Treatment Of Psoriatic Arthritis: Il
Are TNF inhibitors still the gold standard for PsA?
Over the past few years, the FDA has approved three monoclonal antibodies that inhibit the function of interleukin 17 for psoriasis. Two of these agents, secukinumab and ixekizumab , are also approved for psoriatic arthritis .
IL-17 promotes joint inflammation by inducing the release of matrix metalloproteinases and increasing the expression of receptor nuclear kappa-B ligand, which exacerbates cartilage and bone destruction, respectively. Inhibiting the activity of the elevated levels of IL-17 with various monoclonal antibodies has been shown to be beneficial in PsA.
This article will compare the efficacy and safety of the three approved IL-17 blockers in PsA.
Literature Search And Study Characteristics
Initially, 3051 potentially relevant citations were screened, and 2648 remained after duplicates were removed. The flowchart of the literature search is shown in Fig. . After manually searching the reference lists, our literature search finally identified five published articles including six clinical trials with an overall 1733 patients that could be used in this meta-analysis. All studies were phase III randomized, double-blind, placebo-controlled trials. Secukinumab was evaluated in 4 trials of 3 published articles , and ixekizumab was used in two articles in the treatment of ankylosing spondylitis . No data concerning brodalumab therapy in ankylosing spondylitis were published through the date of literature retrieval. The ASAS20/40 response rate of treatment for ankylosing spondylitis at week 16 was reported in all six trials, while the ASAS partial remission rate was described in three trials . Similar large variations were observed for the proportion of male sex, ranging from 52% to 83.7% , and the mean±SD of age, ranging from 40.1±11.6years to 47.4±13.4years . Patient characteristics are detailed in Table . The methodological qualities of all trials are high in light of the clear declaration of the randomization in patient selection, blinding, and outcomes of all patients in their trials.
Fig. 1
Don’t Miss: How To Stop Guttate Psoriasis Spreading
Search Strategy And Selection Criteria
We searched on PubMed, Embase and Web of Science up to March 2020 all studies on digestive paradoxical effects. The following search terms were used : paradoxical effects, interleukin-17 blockers, anti-IL-17, secukinumab, ixekizumab, brodalumab, ixekizumab, inflammatory bowel disease, Crohns disease, ulcerative colitis, spondyloarthritis, psoriasis and ankylosing spondylitis. No language restriction was applied. We focused on full-text articles, although relevant abstracts were considered. In addition, further studies were identified through the accurate evaluation of the reference lists of the assessed manuscripts. Finally, the studies were included in our review on the basis of originality and relevance.
Therapeutic Implications Of Il
The molecular features of IL-17 made it an attractive therapeutic target and specifically as targeted therapy in plaque psoriasis. Currently, three monoclonal antibodies targeting IL-17 are in clinical development brodalumab, ixekizumab and secukinumab.8-13
Brodalumab is a human, anti-IL-17-receptor monoclonal antibody that binds with high affinity to human interleukin-17RA. IL-17RA blockade inhibits the biologic activity of interleukins 17A, 17F, 17A/F heterodimer and 17E .8 Ixekizumab is a humanized immunoglobulin G4 monoclonal antibody9 and secukinumab is a fully human IgG1 monoclonal antibody10-13 that selectively bind and neutralize IL-17A.
Also Check: Dosage Of Methotrexate For Psoriasis
Data Synthesis And Analysis
A meta-analysis was performed for three factors: efficacy evaluation of IL-17 inhibitors compared with that of a placebo by the primary outcome ASAS20 and secondary outcome ASAS40, IL-17 inhibitor efficacy assessment between TNFi-naïve patients and patients who had previous inadequate response or intolerance to an TNFi therapy , and a comparison of the safety profile between IL-17 inhibitors and the placebo. The meta-analyses were carried out using the Mantel-Haenszel method to determine the weight given to each study. A fixed-effects model was used when there was no significant heterogeneity, whereas a random-effects model was used in other cases. This produced a weighted estimate of the risk ratio with a 95% confidence interval , considering the weight of the different samples. The heterogeneity amongst studies was examined on the basis of the Q test . The heterogeneity was qualified by the I2 statistic, ranging from 0 to 100%, in which high values of I2 represent strong heterogeneity. The sources of heterogeneity were explored by producing a Galbraith radial plot. The likelihood of publication bias was evaluated graphically by using sensitivity analysis. All statistical analyses were performed using RevMan statistical software version 5.3 and Stata/MP version 13.0 . p values lower than 0.05 were considered significant.
Treating Psa With Disease
Treatment for PsA involves therapies that target and disrupt the inflammatory pathways causing joint damage. While agents like NSAIDs and steroids may be helpful in mild disease, alternative agents are often needed in more advanced or active disease.
Disease-modifying anti-rheumatic drugs or, DMARDS, are preferred in active disease and can be classified into three main categories:
- biologics biologics
You May Like: Hydrocortisone For Psoriasis Of The Scalp
How To Take And Store
Your biologic medication should be refrigerated before use. Remove your medication 30 minutes before the time that you are going to administer your injection so that the medication can reach room temperature.
Before you administer your injection, you should have an alcohol pad and sterile bandage ready. Wash your hands with soap and water, and swab the skin where you will administer the injection with an alcohol pad to cleanse the skin and reduce the risk of infection.
The frequency of injections depends on the specific type of medication that you are prescribed. Remicade is delivered intravenously through insertion of an IV into a vein and repeated every eight weeks.
All the other types of biologic medications are injected subcutaneously, or under the skin, most commonly in your abdomen or thigh. Some medications require a frequent injection schedule, such as Enbrel every week and Humira, Siliq, and Cimzia every other week.
Other medications require less frequent injections after the initial dose, such as Cosentyx and Taltz every four weeks, Simponi once a month, Tremfya every eight weeks, and Skyrizi and Stelara every 12 weeks.
It is important to follow your dosing regimen as missed doses can reduce the effectiveness of your biologic medication.