Effectiveness Of Apremilast In Special Locations
Currently, apremilast is approved for the treatment of moderate-to-severe psoriasis, but it has shown effectiveness in difficult-to-treat forms of psoriasis, such as scalp, face, palmoplantar, and nail psoriasis, and hence can be considered in these cases.15,23
The ESTEEM 1 AND ESTEEM 2 phase III double-blinded efficacy and safety trials evaluated the efficacy of apremilast in nail and scalp psoriasis.24 Various other studies have also shown the efficacy of apremilast for the treatment of palmoplantar psoriasis25 and nail psoriasis.26
The clinicians in our survey reported the use of apremilast for palmoplantar, scalp, and nail psoriasis, other than plaque psoriasis, as a second-line drug. The panel mentioned its use in nail and palmoplantar types more frequently than in others.
Ology And Study Design
To assess the real-world experience of apremilast use in psoriasis, an expert panel was formed composed of five dermatologists with a minimum of 15 years experience in the management of psoriasis and experience of apremilast prescription for at least 2 years.
The entire survey process consisted of three steps: 1) literature review and questionnaire design 2) the survey of eligible clinical dermatologists and 3) panel discussion for review of results and consensus generation.
Effectiveness Of Apremilast As Maintenance Or Switchover
The panel remarked that apremilast could be added as a maintenance or switchover drug in patients receiving methotrexate or cyclosporine with acceptable control of psoriasis. This strategy is found to be effective in reducing the side-effect profile of other conventional systemic drugs.
However, withdrawal of conventional drugs may be associated with rebound or flares, hence it was cautioned that conventional drugs should be withdrawn slowly over 46 weeks.
Read Also: Herbal Remedies For Psoriasis What Are Our Patients Taking
Clinical Study For Itolizumab In Lupus Nephritis Initiated In India Post Dcgi Approval
- Thu, 23-Dec-2021
Bengaluru, India, December 23, 2021
Biocon Biologics Ltd., a subsidiary of Biocon Ltd. , today announced that U.S.-based Equillium Inc., Biocons partner, has expanded its EQUALISE study in Systemic Lupus Erythematosus and Lupus Nephritis for Itolizumab to clinical centers in India. EQUALISE is a Phase 1b open-label, proof-of-concept clinical study currently studying Lupus Nephritis patients in the Part B portion of the clinical trial.
Equillium has initiated this study across several tertiary hospitals specialised to deal with Lupus Nephritis patients in India after obtaining approval from the Drugs Controller General of India .
Systemic Lupus Erythematosus, or Lupus, is an autoimmune chronic inflammatory disease. The prevalence of SLE in the U.S. has been reported to be between 20 to 150 cases per 100,000. In India, the reported prevalence of SLE is 3.2 per 100,000.
Dr. Sandeep Athalye, Chief Medical Officer, Biocon Biologics, said: We are happy to announce the commencement of our partner Equilliums Phase 1b clinical study in India to evaluate the safety and early efficacy of Biocon Biologics novel antibody, Itolizumab, in treating Lupus Nephritis. In India, approximately 45,000 patients are diagnosed with Systemic Lupus Erythematosus , of which over 20,000 patients have kidney involvement , many of which do not respond to standard available therapy with steroids and immunosuppressive drugs.
About Systemic Lupus Erythematosus / Lupus Nephritis
Traditional Treatments Arent Working
Traditional treatment options for psoriasis include topical creams, corticosteroids, cyclosporine, retinoids, methotrexate, and phototherapy. People with mild to moderate psoriasis can usually manage their disease well with topical treatments. But these treatments often dont work well enough for those with moderate to severe cases. Some treatments may also lose effectiveness over time.
If you have moderate to severe psoriasis and your current treatment regimen isnt working, its time to start considering a biologic. The American Academy of Dermatology suggests taking a biologic agent if you have moderate to severe psoriasis that hasnt improved using more traditional systemic agents or you cant tolerate those treatments because of side effects.
Recommended Reading: Is Psoriasis Skin Disease Contagious
Biologics Treatment For Psoriasis In India
Theres nothing fun about having a flaky, itchy scalp. Some experience small flurries of flakes when their scalp is dry,
Biologics are protein-based drugs derived from living cells cultured in a laboratory. Biologic drugs are a relatively new class of treatment for.
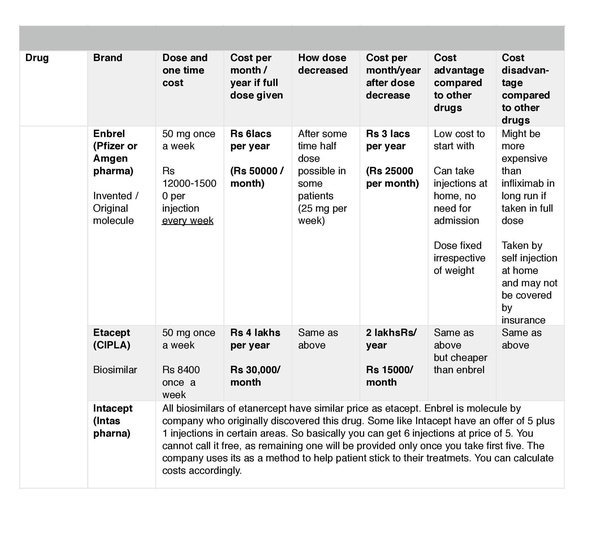
The biologics currently in use for psoriasis in India are etanercept, infliximab and.
The decision to proceed with treatment must be made in.
The biologics currently in use for psoriasis in India are etanercept, infliximab and.
Patients with psoriasis may be considered eligible to receive treatment with.
Hoth Initiates Preclinical Gene Therapy Program with NC State for the Treatment of Asthma and Allergic Inflammation /PRNewswire/ Hoth Therapeutics, Inc. , a biopharmaceutical company focused on unique targeted therapeutics.
Various biologics currently used in the treatment of psoriasis have been listed in . The biologics and biosimilars which.
Dose reduction of biologics adalimumab, etanercept, and ustekinumab was not shown to be as effective as providing the usual doses for treatment of psoriasis, according to study findings published.
Parasramani SG, Pillai J. Biologics in psoriasis: Indian experience. Indian J Drugs Dermatol 2019 5:1-5.
Mumbai: Indian patients of psoriasis, a chronic, autoimmune skin.
The treatment cost for biologics ranges from Rs300,000 to Rs600,000 per.
/PRNewswire/ Allied Market Research recently published a report, titled, Psoriatic Arthritis Treatment Market by Drug.
Your Psoriasis Is Mild But Really Bothers You
Biologics are typically reserved for those with moderate to severe psoriasis, but they could be an option if your psoriasis is greatly affecting your quality of life.
Even if your psoriasis is considered mild, you may have painful plaques on the soles of your feet, your palms, your face, or your genitals. The pain may prevent you from doing your usual activities. In these cases, a switch to a biologic may be justified.
Read Also: How Do They Test For Psoriasis
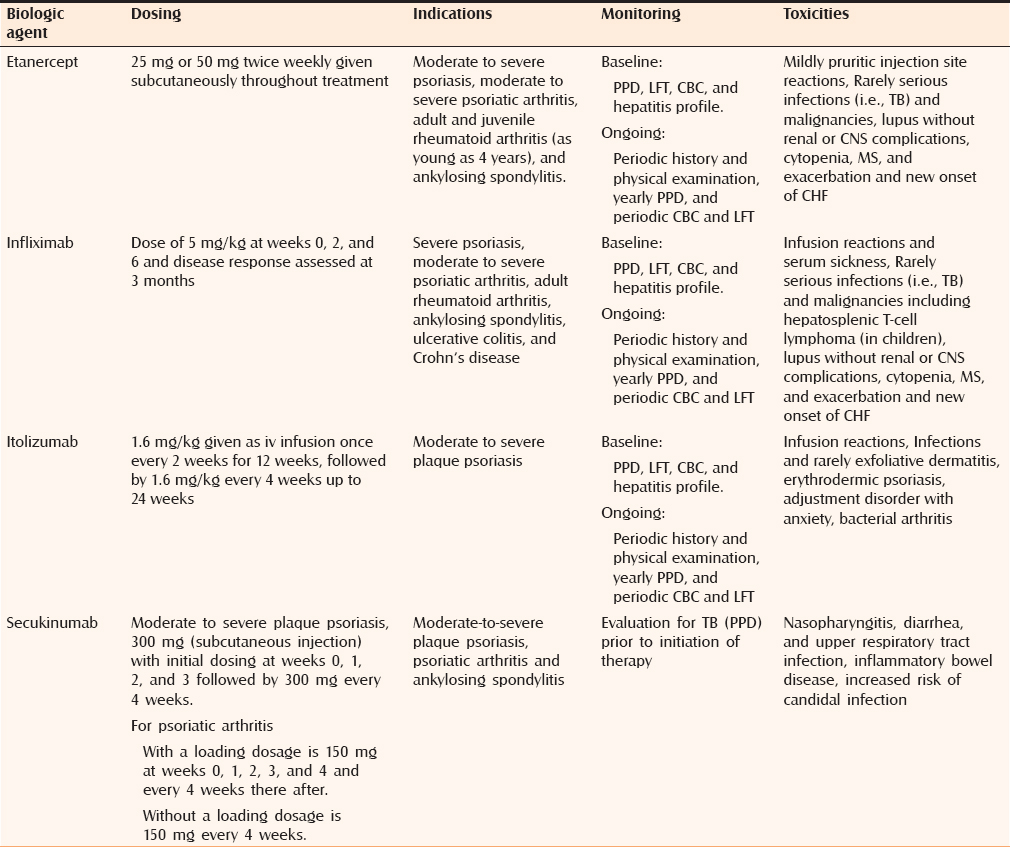
Tumor Necrosis Factor Alpha Blockers In India
TNF plays a central role in the pathogenesis of psoriasis, psoriatic arthritis, and a number of other disease states. TNF is released from cells as a soluble cytokine following cleavage from its cell surface-bound precursor . Both sTNF and tmTNF are biologically active, and bind to either of the two distinct receptors: TNF receptor 1 and TNF receptor 2. This leads to NF-kB activation and or cell apoptosis. In addition, tmTNF can itself act as a ligand to induce cell activation, cytokine suppression, or apoptosis of the tmTNF bearing cell. Soluble forms of the TNF receptors also exist, and by binding and neutralizing sTNF, may act as natural TNF antagonists. There are currently two approved groups of biologic agents that target TNF: anti-TNF monoclonal antibodies , and sTNF receptors . Adalimumab is not currently available in India. Infliximab is a chimeric humanmurine monoclonal antibody Etanercept is a genetically engineered fusion protein composed of a dimer of extracellular portions of human TNFR2 fused to the Fc domain of human IgG1.
Effectiveness Of Apremilast In Combination With Systemic Drugs
According to the survey results, a combination of apremilast is documented with other anti-psoriatic agents, such as methotrexate, acitretin, cyclosporine, and other biologic therapy. This was corroborated by the published literature.16
Methotrexate was found to be most commonly prescribed drug, along with apremilast, followed by cyclosporine. This combination has been previously proven as a good and effective combination for the treatment of psoriasis. It has also been proven that methotrexate and apremilast can be coadministered without any effect on the pharmacokinetic exposure of either agent.17 The panel suggested that the combination of cyclosporine and apremilast could be a viable option in severe psoriasis by virtue of rapid relief with cyclosporine, followed by apremilast continued for a longer duration to maintain remission.
Read Also: Can You Develop Psoriasis In Your 60s
Read Also: Herbal Remedies For Scalp Psoriasis
Who Can Take Biosimilars
All biologics, including biosmilars, are typically prescribed for people with more advanced disease, including individuals with moderate-to-severe psoriasis and active PsA. But each of the three approved biosimilars are indicated for different groups within this population.
You should not take biosimilars if:
- Your immune system is significantly compromised
- You have an active infection
Screening for tuberculosis or other infectious diseases is required before starting treatment with all biologics, including biosimilars.
Maintenance Therapy With Apremilast
In various studies, apremilast has been evaluated as monotherapy or in combination therapy for long-term maintenance in patients with plaque psoriasis.4,6,7 After considering these facts, the experts commented that apremilast should be preferred as long-term maintenance therapy in patients with plaque psoriasis because of its better safety profile, lack of organ toxicity, and reduced need for laboratory monitoring.
Even though the approved dose of apremilast is 30 mg twice daily, a few studies have evaluated the efficacy and safety of apremilast 20 mg twice daily in patients with plaque psoriasis and psoriatic arthritis. In the PALACE trials, apremilast 20 mg twice daily was equally effective compared to apremilast 30 mg twice daily. Ohtsuki et al reported similar results in patients with plaque psoriasis with apremilast 20 mg twice daily.84
Based on these facts, the results of the survey, and their personal experience, the experts commented that a lower dosage of apremilast, such as 20 mg twice daily or 30 mg once daily as long-term maintenance therapy, can be considered in patients who have achieved clearance of their lesions. This strategy may help in reducing the side-effect profile and the cost of the therapy, resulting in better patient compliance and thus improving treatment outcomes.
Recommendations for this section are summarized in Table 4.
|
Table 4 Recommendations on Dose Titration and Maintenance, and Laboratory Monitoring |
Recommended Reading: Does Psoriasis Burn And Itch
Patients At Risk Of Developing Tuberculosis
A high prevalence of latent TB infection has been reported in autoimmune disorders such as psoriasis.71 In addition, conventional and biologic therapies such as TNFi are associated with an increased risk of developing opportunistic infections such as TB.72 Hence, the management of such patients becomes challenging.
A pooled analysis of landmark clinical trials in psoriasis did not report reactivation of TB with apremilast.41 In the post-marketing report of 117,728 psoriasis patients who were exposed to apremilast, only three patients reported TB and none of the patients discontinued therapy with apremilast.49 Therefore, TB screening and monitoring are not recommended routinely in patients receiving apremilast.41
Based on these facts, apremilast can be considered as a therapeutic option in patients with TB or those who are at risk of developing TB. However, rifampicin, being a strong inducer of CYP3A4, may result in a loss of efficacy of apremilast.30 Thus, the concurrent use of rifampicin with apremilast is not recommended.
Discontinuation Of Biologics Due To Adverse Effects
The data on discontinuation due to adverse effects are reported in Table . There were 4 studies providing data for etanercept, 5 studies for adalimumab, 2 studies for infliximab, and 4 studies for ustekinumab. The meta-analysis on the drug survival as to discontinuation due to adverse effects is illustrated in Fig. and the forest plots are shown in Supplementary Fig. . Ustekinumab was the least frequently associated with discontinuation due to adverse effects. On the other hand, infliximab was most frequently associated with discontinuation due to adverse effects.
Read Also: Best Shaving Cream For Psoriasis
Your Current Therapy Is Causing Side Effects
Psoriasis treatments like cyclosporine, corticosteroids, and methotrexate are known to cause side effects like mouth sores, nausea, upset stomach, and even skin cancer.
Biologics work in a more selective way than other psoriasis treatments. They target specific proteins in the immune system that have been proven to be associated with psoriasis. For this reason, they have fewer side effects than less targeted treatments.
Biologics can still cause side effects, but they tend to be less severe. The most common side effects are minor irritation, redness, pain, or a reaction at the site of injection. Theres also a slightly higher risk of serious infections.
Another possibility is to take a combination of your current therapy along with a biologic. By combining treatments, you can improve the efficacy of your treatment and lower the dose. This helps to decrease side effects. certolizumab pegol , etanercept , adalimumab , and infliximab have been shown to be safe and effective when taken with methotrexate.
Biologics For Psoriasis In India
Laboratory Monitoring While Using Apremilast
In clinical trials on the efficacy and safety of apremilast, laboratory parameters did not show any significant derangements. Also, there was no evidence of cumulative or organ-specific toxicity.50 According to the prescribing information for apremilast, no laboratory monitoring is required.41 However, in a consensus study on the use of apremilast in psoriasis, published in 2020,8 and a real-world study by Mayba and Gooderham,7 it was recommended that laboratory monitoring should be carried out only in selected cases.
Based on the evidence, clinical experience, and survey results, the experts recommended that:
- Laboratory monitoring in the form of complete blood count should be conducted at least once or twice a year in patients receiving apremilast. In patients on combination therapy, laboratory monitoring guidelines of the other agent should be adhered to.
- Additional laboratory monitoring should be conducted according to the patients underlying disease.
- From the above findings and less rigorous pre-screening, apremilast therapy is more convenient than other conventional therapies.
Common Side Effects of Apremilast
Based on the above findings and clinical experience with the use of apremilast, the experts opined that most of these AEs are mild in severity and resolve with time and appropriate pharmacotherapy, as also noted in the published literature.78
Dose Titration of Apremilast
Don’t Miss: Best Natural Cream For Psoriasis
Less Risk Of Infection
The research team used data from two insurance claims databases that included more than 250 million people in the United States.
They tracked the incidence of serious infection requiring hospitalization in 107,000 people with psoriasis who had a prescription claim for one of seven systemic drugs approved to treat moderate to severe psoriasis.
The drugs included acitretin and methotrexate. The researchers also looked at the biologic drugs adalimumab, etanercept, ustekinumab, and apremilast.
One of the side effects that patients and physicians are the most concerned about with these medications is a potential increased risk of infection, Dommasch said.
She says the study was limited so far as they were unable to identify many users of infliximab, which is given as an intravenous medication.
Therefore its often not coded in databases as a prescription fill, which is how we identified users of the systemic medications, she noted. We also werent able to look at baseline psoriasis severity across the different medications, which also may impact the risk of infection.
Patient Population And Interventions
The cost-effectiveness analysis was conducted in adult PsA patients of at least 18 years of age, with active disease in spite of treatment with NSAIDs, csDMARDs, and/or TNFi. The baseline patient characteristics were derived from the FUTURE 2 study , as shown in Additional file : Table S1. Patients were classified into three groups based on prior exposure and response to biologics and psoriasis severity as, biologic-naïve without moderate to severe psoriasis, biologic-naïve with moderate to severe psoriasis, and biologic-experienced . Biologic-naïve patients without moderate to severe psoriasis received 150 mg of secukinumab, while biologic-naïve patients with moderate to severe psoriasis and biologic-experienced patients received secukinumab 300 mg. The secukinumab dosing regimen was based on the approved marketing authorization guidelines and is reflective of the common prescription practice of rheumatologists in Finland. All patients are assumed to continue receiving concomitant standard of care treatments. Information related to treatment regimens like dosing and frequencies are provided in Additional file : Table S2.
You May Like: How Do You Get Psoriasis On The Scalp
New Era Of Psoriasis Treatment
In the 1960s and ’70s, new info about how the immune system — your body’s defense against germs — plays a role in psoriasis led to several new treatments. Drugs like corticosteroids, cyclosporine, and methotrexate became mainstays for managing the disease. For the next few decades, though, advances in treatment slowed down.
Thanks to recent progress in research, that’s history.
Scientists studying other autoimmune diseases found new insights about the immune system. It turns out that some of the problems in those conditions are active in psoriasis, as well.
The new info brought treatments that target specific areas of your immune system. Called biologics, these drugs launched a new era of psoriasis treatment. New biologic therapies work well to treat psoriasis, and other new treatments are close to FDA approval.
How Biologics Are Going To Be The Driving Force For Indian Pharma
- The author of this article is Dr R.B Smarta, Chairman and Managing Director, Interlink
As the healthcare scenario is experiencing the paradigm shift from cure to care, preventive health has received generous importance and hence the biologics. Growing demand for vaccines, monoclonal antibodies and biosimilars are creating excellent business space for Indian pharma.
Indian pharmaceutical sector has great potential to earn the identity of global hub for manufacturing biologics. The ability to invent globally competitive, affordable and novel vaccines and biosimilars is emerging as one of the greatest driving forces for Indian pharma. As the industry is shifting from chemical-based drugs to biosimilars and biologics, this scenario presents excellent opportunities for Indian pharma industry in the space of life sciences and biotechnology.
Till September 2019, India received over 98 biosimilars approvals in the domestic market, more than any other country. Moreover, the approvals which we are receiving for biosimilars in regulated market, further boosting the confidence and willingness of Indian pharma players to contribute in global market shares. Owing to the rising domestic demand, potential and investments in biologics in India, according to the reports, more than 40 biosimilars reached clinical development stage in India which is far more than that of United states and similar to European Economic Areas.
Vaccines establishing strong presence
Also Check: Psoriasis On Hands And Feet Treatment
Psoriasis Weight And Nutrition
No single diet or food will treat or prevent psoriasis. And you can get this condition at any size. Still, there are some signs that nutrition and weight do affect it — just as they affect many other conditions.
Body fat fuels inflammation. Doctors have known for a while that losing weight can reduce psoriasis symptoms and help your medicines work better at clearing your skin. The open question is: What’s the best way to lose weight?
In studies, people with psoriasis who trimmed off as little as 5% of their weight by following a low-calorie diet had clearer skin.
The traditional Mediterranean diet can also help lower inflammation, with foods like fish, fruit, vegetables, nuts, and olive oil. Researchers are looking at whether that might help make psoriasis less severe.
One small, short study offered a very low-calorie keto diet, followed by 6 weeks of a traditional Mediterranean diet. All the people in the study were overweight or obese and had psoriasis but werenât taking medication for it. They lost weight, and their psoriasis improved. Itâs not clear if that was because of the weight loss, the types of food they ate, or both things. More research will be needed to see exactly what worked.