How Safe Are The Drugs
Most of what is known about side effects of the biologics comes from trials of people with rheumatoid arthritis, Crohns disease, or other ailments. The risk of experiencing a side effect for people with psoriasis appears to be less because combination therapy with methotrexate and other medications that suppress the immune system were not used in psoriasis clinical trials.
The risk of experiencing side effects is an important factor to consider when choosing to take a biologic drug.
Also Check: How Many People Have Plaque Psoriasis
Relationship Between Biosimilars And Biologics
Biosimilars are highly similar to their biologic reference product. All biologics, including biosimilars:
- Target specific parts of the immune system rather than impacting the entire immune system
- Are given as an injection or IV infusion
To be approved as a biosimilar for a particular reference biologic, the biosimilar must be:
- Highly similar to the reference product and cannot have any clinically meaningful differences in terms of safety or efficacy
- Approved for the indication and condition for which the reference product is approved
- Be given the same form and in the same dosage as the reference product
- The same mechanism of action as the reference product, which means it works the same way in the body
An interchangeable biosimilar must meet the biosimilar standard plus an additional standard that the treatment will produce the same clinical result as the reference product in any given patient. If a biosimilar is approved as interchangeable, a pharmacist may substitute it without letting your prescribing health care provider know, in some states.
Keep in mind that biosimilars are not exact copies of their biologic reference product. Biologics are large and complex molecules from living sources that cannot be exactly copied.
Many considerations go into a treatment decision. Always speak with your health care provider about the potential risks and benefits of a treatment recommendation.
When Should Biological Agents Be Used
Due to the high cost of these medicines, their use is limited to patients with moderate to severe psoriasis where:
- All other treatments have failed
- Side effects of other treatments become intolerable or toxicity has occurred
- Concurrent diseases such as congestive heart failure or liver disease preclude the use of currently available systemic therapies.
In New Zealand, infliximab, adalimumab, etanercept and secukinumab are funded by PHARMAC for some cases of severe psoriasis on Special Authority application.
Recommended Reading: Best Uv Light For Psoriasis
Skyrizi Is A Biologic That Was Approved For The Treatment Of Moderate
What is Skyrizi?
Skyrizi was approved by the FDA in April 2019 for the treatment of moderate-to-severe plaque psoriasis in adults. Skyrizi was approved by the FDA in January 2022 for the treatment of active psoriatic arthritis in adults.
For both plaque psoriasis and PsA, Skyrizi is given by injection under the skin at week 0 and week 4, and then every 12 weeks afterwards.
To learn more, please visit the Skyrizi website.
How Biologic Drugs Are Administered
Biologic treatments are administered by injection or IV infusion because they consist of large molecules that cannot be properly absorbed orally. The compounds are also complex and fragile, making them unstable in the gastrointestinal system.
Injection or infusion, depending on the specific drug, can be administered in a clinical setting. Many biologic drugs that can be absorbed under the skin, or subcutaneously, can also be self-injected at home.
Researchers are aiming to develop drugs that work like biologics, but which could potentially be taken orally, Dr. Kimball said. We still have areas of research for oral treatments that are potentially more convenient and maybe less expensive over time, she explained. So there’s some good work going on and some new approaches there.
Don’t Miss: How To Remove Psoriasis Black Marks
Discontinuation Of Biologics Due To Loss Of Efficacy
The data on discontinuation due to loss of efficacy are shown in Table . There were 5 studies reporting data for etanercept, 6 studies for adalimumab, 3 studies for infliximab, and 5 studies for ustekinumab. The meta-analysis on drug survival as to discontinuation due to loss of efficacy from year 1 to 4 is shown as Fig. and forest plot is show in Supplementary Fig. . The drug survival rate fell from 0.80 at year 1 to 0.47 at year 4 for etanercept, from 0.88 to 0.57 for adalimumab, from 0.87 to 0.51 for infliximab, and from 0.93 to 0.79 for ustekinumab. Etanercept was the biologics most commonly associated with discontinuation due to loss of efficacy and ustekinumab was the least one.
Table 1 Studies reporting discontinuation of biologics due to loss of efficacy or adverse effects.
Tremfya Is A Biologic Approved For The Treatment Of Psoriasis And Psoriatic Arthritis Tremfya Was Approved By The Fda In 2017 For Adult Patients With Active Psoriatic Arthritis
What is Tremfya?
Tremfya was approved by the FDA in July 2017 for the treatment of moderate-to-severe plaque psoriasis in adults. In July 2020, the FDA also approved Tremfya to treat adults with active psoriatic arthritis.
Tremfya is given by injection under the skin at week 0 and week 4, and then every 8 weeks afterwards.
To learn more, please visit the Tremfya website.
Also Check: What Causes Psoriasis On Legs
Sticking To Your Treatment Plan
Treating PsA takes a lot of work, and it can be hard to keep up with all the aspects of your care. The Psoriasis Foundation says the main reason PsA treatments fail is that people don’t stick to them properly.
Biologic therapy comes with some challenges, including high costs, fear of side effects, not feeling better quickly enough, or the timing of treatment might be inconvenient. It is also not uncommon for people to feel wary of biologics.
But your healthcare provider has prescribed biologic drug therapy because they have determined the benefits outweigh the risks. And, fortunately, most of the newer biologics are effective and safe to use.
Stopping treatment, regardless of the reason, is never a good idea. You will likely find yourself with increased PsA symptoms and flares as soon as you stop using your biologic or other treatments. The decision to stop or reduce treatment should be made between you and your provider.
What Are The Potential Side Effects Of Biologics And How Can Someone Manage Them
The main side effects that biologics can cause include infections and malignancies.
While reducing inflammation in the skin is good for psoriasis, blocking the immune system which defends the body from infections and combats cancerous cells can potentially lead to adverse effects.
If the immune system does not protect the body from infections and cannot recognize and fight off abnormal cells as well as usual, a person may have a greater risk of infections and malignancies.
Besides these risks, TNF blockers have been associated with the development of multiple sclerosis, or MS.
Also, IL-17 blockers have an additional warning about a potential increased risk of inflammatory bowel disease, or IBD.
While the potential adverse effects may be worrisome, they are extremely rare. With regular follow-up visits to a dermatologist, these drugs are safe to use.
The dermatologist will examine the persons skin, assess their medical history for any potentially concerning symptoms, and perform blood monitoring.
Read Also: How To Deal With Psoriasis On Scalp
Also Check: What Is Light Therapy For Psoriasis
Biological Therapy Treatment For Psoriasis
There are many factors that could contribute to itchy skin allergic reactions, insect bites, or dryness. And, that dry feeling is often exacerbated by age. However, it could also be a sign of an underlying medical condition that causes skin inflammation, such as psoriasis.
Biological therapy can help relieve symptoms of psoriasis by suppressing the part of the immune system that causes this inflammation. It can also attack proteins that promote the growth of abnormal cells which is a root cause of the condition.
Comorbidities Special Populations Idiosyncrasies Influence Decisions
byCharles Bankhead, Senior Editor, MedPage Today March 6, 2019
WASHINGTON — Knowing when not to use a biological agent to treat psoriasis can help optimize outcomes for many patients, a psoriasis specialist said here.
Reasons for considering alternatives to biological therapy vary from valid clinical issues to demographic considerations to personal preferences and idiosyncrasies. At one time, patients’ aversion to needles made adherence to injectable therapies problematic, although that issue has subsided, Russell Cohen, MD, of Mount Sinai Health System in New York City, said during a forum on biologic therapy at the American Academy of Dermatology meeting.
In contrast, direct-to-consumer advertising has emerged as a major influence on patients’ perceptions about therapies, contributing to opposition to biologic therapy that lacks a scientific base but nonetheless makes sense to the patient.
“I had a patient who came in just yesterday 72 years old, total-body psoriasis,” said Cohen. “He had seen many dermatologists, and nothing made it better. He said, ‘I’ve heard that you’re the guy, you’re the biologic guy.’ We talked about everything and he was all ready to do it. I brought in my biologic coordinator, and I said, ‘OK, let’s do it.'”
“He said, ‘I can’t.’ I asked why not, and he said ‘Because my mother said they’re dangerous.’ His 97-year-old mother told him not to take a biologic.”
Comorbid Conditions
Special Populations
Disclosures
Primary Source
Also Check: Get Rid Of Psoriasis On Face
Creams Vs Biologics In Treating Psoriasis
When the life cycle of your skin cells speeds up and goes haywire, youve gotpsoriasis, and youre one of more than8 million Americans that suffer from the condition. Theres no cure for psoriasis, but there are many treatment options that limit the impact of outbreaks, since psoriasis tends to come and go in many patients.
Medical science doesnt know the precise causes of psoriasis, which has many different types, but theyre all linked to immune system dysfunction, where certain components mistakenly attack normal skin cells, as though fighting an infection or healing a wound. New skin cells are quickly overproduced, and the characteristic scaly patches of psoriatic skin develop.
Risk Of Bias Assessment
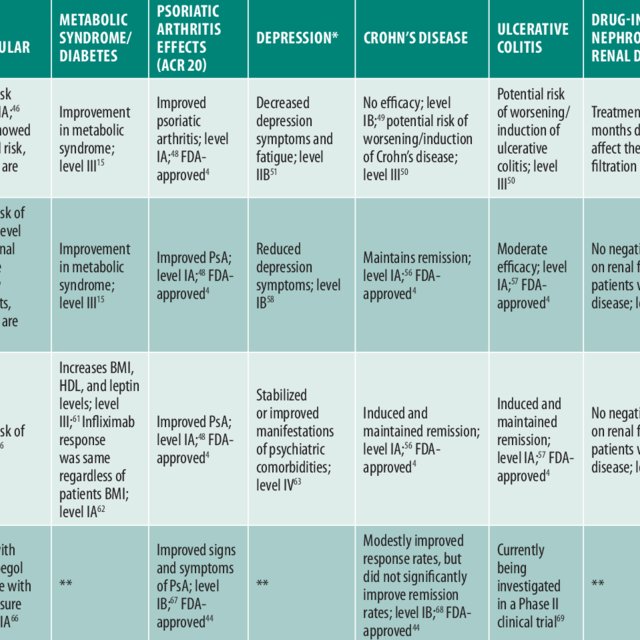
The NOS uses three categories to evaluate the risk of bias of cohort studies. As to the selection category, four items are evaluated, including the representativeness of exposed cohort, selection of non-exposed cohort, ascertainment of exposure, and outcome of the interest not present at start of study. Regarding the comparability category, the comparability of cohorts was assessed. In the outcome category, two items were rated: follow-up duration and adequacy of follow up of cohorts. A study could be awarded up to one star for each numbered item within the selection and outcome categories and up to two stars for comparability. The highest quality studies are awarded up to nine stars.
Read Also: Mg217 Psoriasis How To Use
What Are Biological Treatments
In the strictest dictionary definition, a biological treatment or biologic drug would be any drug that is a biological product this is true for most drugs, as they are extracted from plants, animals, and fungi, or developed through bioengineering.
In actual use, the terms biological treatment and biologic refers to bioengineered monoclonalantibodies. These are made by laboratory animals against a target antigen. The antibodies are monoclonal, meaning :
- They are clones of each other
- The entire clone set attaches to the same type of epitope on the target antigen.
How Do Biologics Work To Help Treat Psoriasis
As we mentioned, drugs like these work because they target a specific part of the immune system thats involved in psoriasis, Dr. Cheng explains. But they accomplish that in slightly different ways.
Biologics that target TNF-alpha are generally older drugs , and because TNF-alpha is involved in a lot of normal bodily processes outside of psoriasis, targeting it could come with more side effects than newer options. Specifically, TNF-alpha is a type of protein called a cytokine, and it has actions all over the body related to infections and inflammation. Thats why, in addition to helping treat the symptoms of psoriasis, drugs that modulate TNF-alpha can also be helpful in treating conditions like inflammatory bowel disease and rheumatoid arthritis.
Those newer optionsbiologics that target IL-17 or IL-23 are working on parts of the immune system that seem to play a large role in the formation of psoriasis plaques. So targeting them is less likely to affect the rest of your body than a TNF-alpha biologic might. Interleukins, another type of cytokine, are produced by white blood cells, which play a crucial role in the bodys immune responses. But different interleukins have different jobs and pathways in the body. Although IL-17 and IL-23 do seem to have minor roles in fighting infections, Dr. Cheng says, their most major role seems to be in psoriasis. Still, no biologic treatment is going to be 100% specific, Dr. Lipner says.
Recommended Reading: Fluocinolone Acetonide Topical Oil For Scalp Psoriasis
What To Expect During Biological Treatments For Psoriasis
Our staff is prepared to assist you in seeking expedited insurance approval in order for you to receive your therapy.
Most biologics are shots that are given subcutaneously under the skin. Patients may choose to perform their injections at home or come to our office for therapy. The frequency of injections will vary depending upon the medication. Your dermatologist will provide you with specific instructions on how to properly undergo treatment for the best possible results.
Pbac Consideration Of The Draft Review Report
The Draft Report, including PBAC sub-committee advice and stakeholder comments, was provided to the PBAC for consideration in April 2018. The PBAC Minutes for this item are now available. Parts of the PBAC Minutes have been redacted due to commercial-in-confidence information.
PBAC Minutes pertaining to the Draft Review Report for the Post-market Review of the use of biologics in the treatment of severe chronic plaque psoriasis.
Considering the findings from Terms of Reference 1, 2 and 3, the PBAC recommended a review of the cost-effectiveness of biologics for severe chronic plaque psoriasis under Term of Reference 4.
You May Like: Early Signs Of Psoriasis On Elbows
Biological Agents For Other Types Of Skin Disease
Other biological agents used for severe skin diseases include:
- Dupilumab , which blocks the IL-4 receptor alpha subunit and is used for atopiceczema
- Rituximab , which is a CD20 antagonist and was initially used for B-cell lymphoma and now for rheumatoid arthritis and granulomatous polyangiitis
- Anakinra , which is an interleukin -1 antagonist registered for rheumatoid arthritis but also found to be helpful for autoinflammatory syndromes such as Schnitzler syndrome, cryopyrin-associated periodic syndrome and adult Still disease
- Omalizumab , which blocks the high-affinity receptor binding site on human immunoglobulin E and is used for asthma and chronic spontaneous urticaria in adolescents and adults > 12 years
- Lanadelumab is a monoclonal antibody that inhibits kallikrein and is used to treat hereditaryangioedema.
There are many other promising biological agents under investigation for skin conditions.
How Do Biologics Work
Biologics are sometimes called biologic response modifiers because they change how certain systems in the body act or respond.
Biologics are given by an injection or through an intravenous infusion into your blood vessels.
They cant be taken by mouth because theyre not strong enough to survive stomach acid. There are also barriers to absorption of the biologic in the gastrointestinal tract.
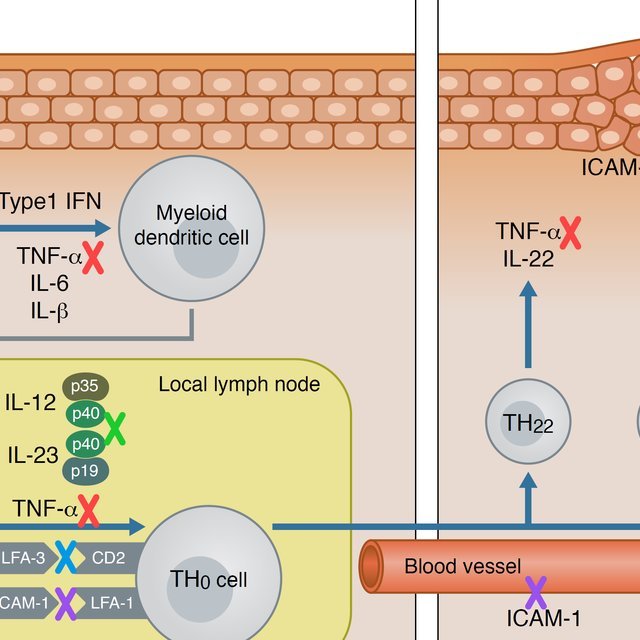
Biologics for psoriasis work by blocking the immune system from making specific cytokines inflammatory proteins that trigger this skin condition. Psoriasis biologics target cytokines produced by two main immune system pathways: Th1 and Th17.
Don’t Miss: Is Manuka Honey Good For Psoriasis
What Makes Someone A Candidate For Biologics And Who Should Avoid Them
Biologics are appropriate for people with moderate to severe psoriasis. In some cases, this refers to psoriasis that affects more than 10% of the bodys surface area.
People with psoriasis that affects less of the skin may still receive biologics. The affected areas may be unique and significant for example, psoriasis affecting the hands can be debilitating, even though it only covers a small percent of the total body surface area.
People who should not receive biologics include those with active cancer, an active infection , and individuals who are systemically unwell, in general.
Biologic Treatment Of Psoriatic Disease
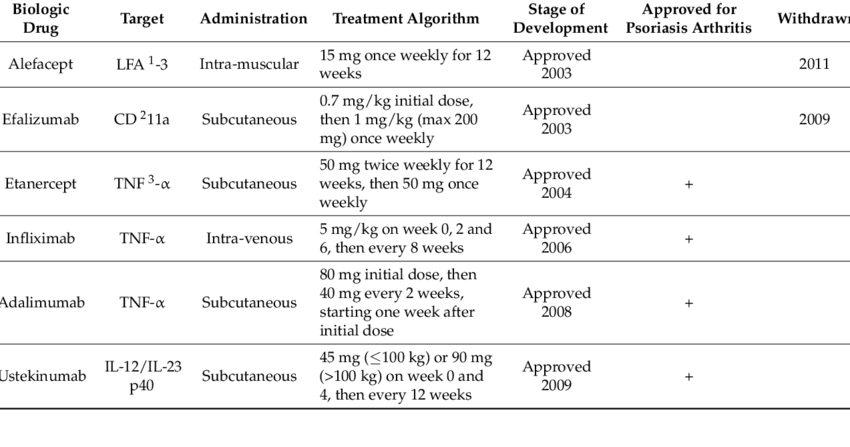
Many different biologic drugs have been approved by the U.S. Food and Drug Administration for treating moderate to severe psoriatic disease. They are not considered a first-line treatment because of their cost and side effects.
While biologics are usually prescribed with a DMARD, they can also be prescribed alone. When a person with disease starts taking a biologic drug, they will also remain on their current treatment plan, which may include non-steroidal anti-inflammatory drugs , corticosteroids, and/or DMARDs.
Also Check: Get Rid Of Psoriasis For Good
Humira Is A Biologic Approved For The Treatment Of Moderate
What is Humira?
Humira is a TNF-alpha inhibitor approved by the FDA in October 2005 for use in adult patients to treat active psoriatic arthritis and in January 2008 to treat moderate-to-severe psoriasis. It is also approved to treat rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, hidradenitis suppurativa, and uveitis.
For adult patients with psoriatic arthritis, Humira is administered every other week. For adult patients with psoriasis, Humira is administered at week 0 and then every other week starting one week after initial dose. Humira is administered by injection under the skin.
To learn more, please visit the Humira website.
List Of Biologics To Treat Psoriasis
Non-biologic DMARDs These medicines slow the disease process by modifying the immune system. Methotrexate is the most commonly prescribed non-.
A list of common psoriasis medications was generated and each medication was divided into various categories for comparison: systemics/biologics, topical noncorticosteroids, and topical.
Learn about how long you will have to treat your psoriasis. The AADs Coronavirus Resource Center will help you find information about how you can continue to care for your skin, hair, and nails. To help care for your skin during the corona.
Biologic treatments for psoriatic arthritis. Biologic medications are specifically designed to mimic chemicals that are naturally found within the human body, and act to correct something that is going wrong. A number of biologic treatments are available to treat psoriatic arthritis. There are various criteria for when biologic treatments can.
Even before the recent development of biological agents, a long list of.
Even prior to the development of biologicals, the treatment of psoriasis was.
Psoriasis is controllable with medication. Psoriasis is not curable. There are many promising new therapies, including newer biologic drugs.
Patients with psoriasis have reported that glycerin, an inexpensive, harmless, slightly sweet liquid high on the list of.
heart disease. Biologics used to treat psoriasis.
medical need and leverages its biologics manufacturing expertise.
Recommended Reading: How Do You Treat Psoriasis Of The Skin