How Safe And Effective Is Stelara
Stelara has been used to treat psoriasis in the UK since 2009. Real-world safety and effectiveness data is being compiled by the British Association of Dermatologists Biologics Interventions Register . It is recommended that all people taking biologic treatments for their psoriasis should be asked for their data to be included in this register.
Over Time A Biologics Effectiveness Might Diminish
After months or years of taking a certain biologic, it may become less effective for you. Thats because your immune system may develop antibodies to the drug, particularly if you missed any doses. If this happens, you might notice plaques gradually coming back, and your doctor may recommend switching to another biologic.
Side Effects And Risks
Stelara and Humira both contain different drugs. Therefore, the medications can cause very different side effects. Below are examples of these side effects.
More common side effects
These lists contain examples of more common side effects that can occur with Stelara, with Humira, or with both drugs .
- Can occur with Stelara:
- redness, pain, or swelling at your injection site
- headache
Serious side effects
These lists contain examples of serious side effects that can occur with Stelara, with Humira, or with both drugs .
- Can occur with Stelara:
- posterior reversible encephalopathy syndrome
- lung problems that are caused by inflammation in your lungs
- possible increased risk of cancer
- severe allergic reaction
Read Also: How To Tell If You Have Psoriasis
Drug Forms And Administration
Stelara and Humira both come as a liquid solution thats given as a subcutaneous injection.
For people taking Stelara to treat Crohns disease or ulcerative colitis, their first dose is given by intravenous infusion . The infusion lasts for at least 1 hour. The rest of their doses are given by subcutaneous injection.
Stelara and Humira can both be given as injections at your doctors office or clinic. They can also be self-injected at home, after your healthcare professional has shown you how to inject the drugs.
Stelara can be injected under the skin of your upper arms, thighs, belly, or buttocks. Humira can be injected under the skin of your thighs or belly.
Stelara comes as a disposable prefilled syringe thats meant to be used once. It also comes as a single-dose vial that youll inject using a syringe and needle. Stelara also comes in a single-use vial thats used for injections given by healthcare professionals.
Humira comes as prefilled, single-dose pens and syringes. It also comes in a single-use vial thats used for injections given by healthcare professionals.
How Is Stelara Administered
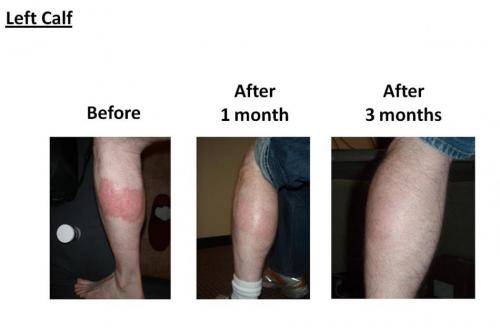
Patients take Stelara as an injection into the upper arm, buttock, thigh, or abdomen. Some patients may be able to inject themselves at home if they are trained by a healthcare provider. It is best to inject the medicine into a different area of skin than the previous injection. Stelara should be refrigerated when not in use.
Most patients will receive the second injection 4 weeks after the first one, and then a single dose every 12 weeks after that. It will usually take around 28 weeks for Stelara to have its full effect3. For adolescents, weight-based dosing will be used.
Don’t Miss: Does Guttate Psoriasis Leave Scars
Keep An Open Line Of Communication With Your Provider
Let them know about side effects, reemerging symptoms or any changes in your health while youre taking Stelara. Patients should also make sure that they ask a lot of questions of their physicians when theyre starting these drugs, says Isaacs, so that they really understand whats happening.
DISCLOSURE: Dr. Isaacs is on a data safety monitoring board for a Janssen new drug study.
Medical reviewer and Oshi physician-partner Matthew J. Hamilton, MD is an Assistant Professor of Medicine at Harvard Medical School and a specialist in Gastroenterology, Hepatology, and Endoscopy at Brigham and Womens Hospital Crohns and Colitis Center in Boston. He is a leading member of the research team at the BWH Crohns and Colitis Center, and has garnered national recognition for his research into the underlying inflammatory processes of IBD.
Oshi is a tracking tool and content resource. It does not render medical advice or services, and it is not intended to diagnose, treat, cure, or prevent any disease. You should always review this information with your healthcare professionals.
What Else Should I Know
The Ustekinumab pen or pre-filled syringe must be refrigerated in its original container. Do not freeze this medicine.
You must continue your regular visits to the rheumatologist. Your doctor will monitor you for any improvements in your disease and for any signs of infections. Lymphoma and other malignancies, some fatal, have been reported in children and adolescent patients treated with TNF blockers, including Ustekinumab.
A Federal Drug Administration approved medication guide can be found at:
Recommended Reading: Is Eating Oatmeal Good For Psoriasis
What Vaccines Can I Get During Stelara Treatment
Stelara makes your immune system less able to function. Certain vaccines are made from weakened forms of a virus. Because your immune system cant fight off the virus very well, you shouldnt get live vaccines during Stelara treatment. Doing so can put you at risk for developing the infection that the vaccine is meant to prevent.
Your doctor may recommend that you have all of the vaccines you need before you start Stelara treatment. This includes both live and non-live vaccines, with the exception of the Bacillus Calmette-Guérin vaccine.
The BCG vaccine is meant to prevent tuberculosis . Its more commonly given to people who live outside of the United States. You shouldnt start taking Stelara for at least 1 year after youve received a BCG vaccine.
If you receive any non-live vaccines while youre taking Stelara, its possible that your immune system wont have the right response to the vaccine. This means that the vaccine might not be able to prevent the infection that its meant to prevent.
Talk with your doctor to make sure you are up to date on all of your vaccinations before you start using Stelara.
How Long Will Stelara Take To Work
It can take a number of weeks before a persons psoriasis or psoriatic arthritis improves on Stelara. If considerable improvement is not seen in four months, treatment with Stelara will be stopped. If this happens, a Dermatologist or Rheumatologist should discuss the next available options with you – there are a number of other biologic or systemic treatments that can be tried if Stelara does not work.
Recommended Reading: Can You Donate Blood If You Have Psoriasis
Dosage For Crohns Disease
The usual dosage of Stelara for adults with Crohns disease is 90 mg given once every 8 weeks. Stelara is given as a subcutaneous injection. When treating Crohns disease, this is called your maintenance dose.
However, the first dose of Stelara that youll receive is called an induction dose. Its given as an intravenous infusion . This infusion lasts at least 1 hour.
The usual dosage for the loading dose is based on your body weight, as follows:
- for adults who weigh 55 kilograms or less, the loading dose is 260 mg
- for adults who weigh between 55 kilograms and 85 kilograms , the loading dose is 390 mg
- for adults who weigh more than 85 kilograms, the loading dose is 520 mg
Every dose of Stelara that you receive after your loading dose will be given as a subcutaneous injection.
Stelara For Crohns Disease
Stelara is FDA-approved to treat moderate to severe Crohns disease in adults.
Crohns disease is a type of inflammatory bowel disease. It causes inflammation in your gastrointestinal tract.
With Crohns disease, you may have:
- diarrhea
- pain and cramps in your belly
Effectiveness for Crohns disease
One clinical study looked at effectiveness after 8 weeks of treatment. In this study, clinical remission was reached in 14% to 21% more people who took Stelara than in those who took a placebo .
Clinical remission can also be defined based on a scoring system that measures how severe peoples Crohns disease symptoms are. Lower scores show fewer Crohns disease symptoms. If you have a score of less than 150 points, youre considered to be in remission. The highest possible score is 1,100.
In these 8-week studies, between 18% and 26% more people taking Stelara than those taking the placebo had a decreased score of at least 100 points.
You May Like: Best Soap For Psoriasis And Eczema
What About Side Effects
The medicine can cause itching or redness near the injection site. If this happens, the discomfort should be mild. If you have pain, swelling, warmth, or discoloration near the injection site, you should contact your healthcare provider.
Allergic reactions may happen. Call your healthcare provider or 911 if you have any signs of an allergic reaction, such as rashes or hives swollen face, eyelids, lips, or tongue and difficulty breathing.
The most common serious side effect is infection. Ustekinumab can lower the bodys ability to fight infection. Be sure to contact your physician if you have any signs of infection, such as fever, fatigue, cough, or red or painful skin. You may have to stop Ustekinumab while being treated for an infection. You may also have to stop if you are planning a surgery.
You will need to have a negative tuberculosis test before beginning Ustekinumab therapy. Your doctor will also check your blood to make sure you do not have Hepatitis B or C.
Make sure your doctor knows if you have any symptoms of heart disease, like shortness of breath when you lie down or exert yourself, swelling or edema of your legs, ankles, and feet, or chest pain or heaviness. This class of drugs may cause your heart disease to get worse.
Tell your doctor if you live or have lived in an area where fungal infections are more common. You may be at higher risk of getting a fungal infection while taking Ustekinumab.
What Is The New Pill For Psoriasis
drugpsoriaticpsoriasisHere are 10 ways to manage mild symptoms from the comfort of your home.
- Topical corticosteroids. These drugs are the most frequently prescribed medications for treating mild to moderate psoriasis.
- Vitamin D analogues.
Also Check: How To Deal With Psoriasis
How Does Stelara Work
Stelara is a monoclonal antibody.
Monoclonal antibodies are man-made proteins that act like human antibodies in the immune system. They are a type of targeted treatment. Targeted treatments attach only to specific proteins in the body.
Stelara binds to the p40 protein subunit that is used by two cytokines, IL-12 and IL-23. Cytokines are signaling substances that help control immunity, inflammation, and the manufacture of blood cells.
- Getting comfortable with Stelara. Janssen Biotech 2016
- Stelara Janssen Biotech, Inc.
- General Information about Stelara. Janssen Biotech, Inc.
- Cush J. Medpage Today News: Biologics Storage: Getting It Just Right Medpage Today. .
- Package leaflet: Information for the user. STELARA 45 mg solution for injection in pre-filled syringe
How To Inject Stelara
Patients often wonder how to best inject Stelara themselves. However, it is important to note that you should not attempt to give any injections without first speaking with your doctor. If your doctor has decided that at-home injections are the best route, then the following video from John Hopkins Rheumatology provides helpful step by step instructions and demonstration.
If you have any questions or concerns about self-injecting Stelara, you should speak to your doctor.
Don’t Miss: What Causes Psoriasis To Get Worse
Dosage For Plaque Psoriasis
Stelara is approved to treat plaque psoriasis in both adults and children ages 6 years and older. The typical dosage for adults is described here.
Stelara is given as one subcutaneous injection on each of the following days:
- your first dose is given on day 1
- your second dose is given 4 weeks later
- your third dose is given 12 weeks after your second dose
- the rest of your doses are given every 12 weeks
The usual dosage of Stelara for plaque psoriasis is based on your body weight and age. In adults with plaque psoriasis, the typical dosage of Stelara for each injection is as follows:
- for adults who weigh 100 kilograms or less, their usual dosage is 45 mg
- for adults who weigh more than 100 kilograms, their usual dosage is 90 mg
Stelara: Side Effects How Its Taken Cost And Mor
- Ustekinumab is a human monoclonal antibody. It is directed against interleukin 12and interleukin 23, naturally occurring proteins that regulate the immune system and immune-mediated inflammatory disorders. In two Phase III trials for moderate to severe psoriasis, the longest > 76 weeks, Ustekinumab was safe and effective
- Humira to Stelara Stelara to Cosentyx And the only thing I was ever told was that I had to wait till the last dose had finished its time. So for Stelara it would be 12 weeks after your last shot before you can move over to Taltz. Welcome to Psoriasis Club by the way
- Find 56 user ratings and reviews for Stelara Subcutaneous on WebMD including side effects and drug interactions, medication effectiveness, ease of use and satisfactio
- istration in 2009 and is also approved for treating psoriatic arthritis 1.. Ustekinumab, the active ingredient in Stelara, is a type of biologic therapy. It is a powerful treatment that affects the way the immune system works to help.
- The use of Tremfya and Stelara in treating moderate to severe plaque psoriasis has been directly compared in a clinical study. Researchers looked at people whose symptoms didnt clear up within.
- Tremfya and Stelara are both injections used to treat plaque psoriasis and psoriatic arthritis in adults. Stelara is prescribed for other conditions, too, such as ulcerative colitis and Crohns.
Don’t Miss: Natural Products For Scalp Psoriasis
Stelara For Plaque Psoriasis
Stelara is FDA-approved to treat moderate to severe plaque psoriasis in adults and children ages 6 years and older.
Its approved for use in people who could receive treatment with either:
- systemic therapy ,
- or
Plaque psoriasis is an autoimmune disease that causes plaques on your skin. Plaques are areas on your skin that are raised and may be red- or silver-colored. They may also feel itchy.
Effectiveness for plaque psoriasis
In clinical studies, Stelara was effective in treating adults and adolescents with moderate to severe plaque psoriasis.
After 12 weeks of treatment:
- Up to 73% of adults taking Stelara had very few plaques to no plaques on their skin. Of those taking a placebo , 4% had the same results.
- Almost 70% of adolescents taking Stelara had very few plaques to no plaques on their skin. Of those taking a placebo, 5.4% had the same results.
Also, 77.3% of children ages 6 to 11 years had very few to no plaques on their skin. No one in this age group took a placebo.
These studies also looked at how many people had fewer plaques on their skin after treatment. The studies found that:
- Up to 76% of adults taking Stelara had at least a 75% improvement in their psoriasis symptoms. Of those taking a placebo, 4% of adults had the same results.
- Up to 80.6% of adolescents taking Stelara had at least a 75% improvement in their psoriasis symptoms. Of those taking a placebo, about 11% of adolescents had the same results.
How Long Does It Take For Stelara To Work
If you or a loved one have been prescribed Stelara, you are almost certainly wondering when you can expect to see results. Depending on the condition being treated, the time it will take to notice results can vary. However, the majority of patients report noticing some improvement within three weeks of their first Stelara dose. Below are more details for the different conditions Stelara is used to treat:
- Psoriasis Around three-quarters of patients taking Stelara for psoriasis achieved a PASI75 within twelve weeks of treatment.
MyTherapy: Making Medication Management a Breeze
- Psoriatic Arthritis After six months of treatment, 50% of patients reported an ACR20 and 15% reported and ACR70
- Crohns Disease After three weeks, 50% of patients achieved CDAI70 and after forty-four weeks, 47% of patients were in clinical remission.
- Ulcerative Colitis After eight weeks, 24% of patients achieved clinical remission and by week forty-four 45% of patients achieved clinical remission.
Don’t Miss: What Is The Best Medicine For Psoriasis
Answers To The Most Frequently Asked Questions About Stelara
Stelara, the brand name for ustekinumab is an immunosuppressant drug. It inhibits the effects of chemical substances in the body responsible for inflation. Its most common uses are in treating psoriasis, psoriatic arthritis, Crohns disease, and ulcerative colitis.
Stelara Ok’d For Psoriatic Arthritis
Oct. 4, 2013 — Stelara, an injectable drug already approved to treat psoriasis, is now also approved to treat psoriatic arthritis.
Psoriatic arthritis is an autoimmune disease. The immune system attacks healthy skin cells and joints, causing inflammation. Of the 7.5 million people with psoriasis, about 10%-30% get psoriatic arthritis.
Recommended Reading: How To Cover Psoriasis On Face
Biologics Are Usually Prescribed To Treat Moderate To Severe Psoriasis
Before prescribing any psoriasis treatment, your doctor may identify the severity of your condition in one of three categories, depending on how much of your body is covered in skin lesions:
- Mild: covers less than 3 percent of your body
- Moderate: covers 3 to 10 percent of your body
- Severe: covers greater than 10 percent of your body
Even if psoriasis covers only a small part of your body, it could still be considered severe if it greatly impacts your quality of life.